
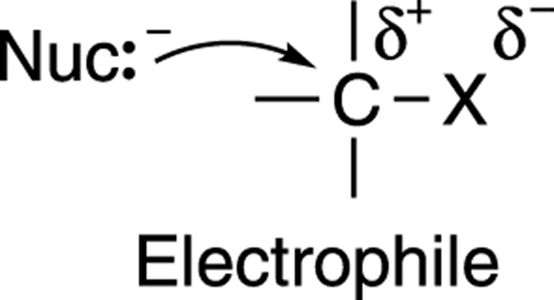
However, when I posted this as an answer in this question, a comment on my answer mentioned that the concept of rate determining step is not applicable and basically useless. Hence for carbon with greater electron deficiency rate and reactivity increases. Attack of nucleophile is faster if the carbonyl carbon is electron deficient. is attack of the nucleophile, which forms a tetrahedral intermediate. Hence rate increases and reactivity increases for stable carbocation.įor nucleophilic addition the r.d.s. Formation of carbocation is faster if the carbocation is stable. I am just finishing high school, and I have been taught that the reason for this is as follows:įor any reaction the overall rate is determined by the slowest step, called the rate determining step (r.d.s).įor electrophilic addition, the r.d.s.
The relation between reactivity order and electron density is opposite in the two cases. more the positive charge (less electron density) on carbonyl carbon, more is the reactivity. when the electron density on carbon atom is more.įor nucleophilic addition on a carbonyl group the reactivity is opposite, i.e. As the positively-polarized carbonyl reacts with (forms a bond with) the nucleophile we pass over a transition state and fall into a second potential well representing the tetrahedral intermediate.It is well-known that for electrophilic addition across an alkene, the reactivity increases when intermediate carbocation is more stable (eg. The energy well for the starting carbonyl compound is shown on the left. In the meantime I welcome everyone to share their thoughts on the matter.īelow is a drawing of the reaction coordinate for nucleophilic attack at a carbonyl carbon. Which I will hopefully be able to prove soon. While I agree with most of this, I do strongly disagree with the last statement. Such calculations might be a poor use of your time and resources. This order of reactivity of carbonyl compounds towards nucleophilicĪttack (we could use water as the nucleophile) - but Martin, I think Of compounds in order to see if his calculated values correlated with
#Reactivity of carbon centered nucleophile with electrophile series
Meaningful if he were to calculate 1) the carbonyl carbon LUMOĬoefficient and 2) the HOMO-LUMO separation for the following series I would be more convinced that his actual numbers are I believe Martin's approach (basically frontier MO) is fundamentallyĬorrect.I like Martin's posts, they make me think - and that is always a good The key points that will be included are stated in ron's answer linked above. I will also later on post an answer to this question using quantum chemical calculations, trying to generalise from example. I am looking here for any model that can be used to explain this reactivity. There might be many concepts out there, that explain this order, the above linked question could only halfway satisfactory answer it in the kind of generality I am looking for. One of the most simplest questions you can ask, how can you rationalise the order of reactivity towards nucleophiles, which is given asĪcyl halide > acid anhydride > aldehyde > ketone > ester ~ carboxylic acid > amide > carboxylate ion This is immediately following ron's answer from Why is a ketone more nucleophilic than an ester?


 0 kommentar(er)
0 kommentar(er)
